High-Risk Kappa Light Chain Multiple Myeloma with Cast Nephropathy: A Case Report
High-Risk Kappa Light Chain Multiple Myeloma with Cast Nephropathy: A Case Report
Abstract
We present a case of a 58-year-old female diagnosed with high-risk Kappa light chain Multiple Myeloma (MM) with cast nephropathy requiring hemodialysis. Notable features include del(17p) and del(13q) cytogenetic abnormalities, successful transition from CyBorD to VTd regimen, and improvement in renal function. This case demonstrates the importance of risk-adapted therapy and treatment modification based on clinical response.
Introduction
Multiple myeloma with cast nephropathy and high-risk cytogenetics represents a therapeutic challenge requiring careful balance of treatment intensity and toxicity management. The presence of del(17p) is associated with poor prognosis, necessitating optimal therapeutic strategies.
Case Presentation
Initial Presentation (Early 2023):
- 58-year-old female with hypertension presented with:
- Easy fatiguability
- Lower limb swelling
- Facial puffiness
- Weight loss and poor appetite
- Initial labs showed severe anemia (Hb 6.3) and renal dysfunction
Diagnostic Workup:
1. Initial Findings:
- High FLC ratio: 4056.5
- Beta-2 microglobulin: >20,500
- Bone marrow: 80% plasma cells
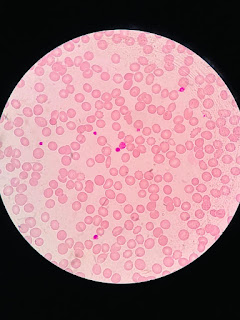
- Kidney biopsy: Cast nephropathy with kappa light chain deposits
- FISH: del(13q), del(17p) - indicating high-risk disease
- Cytogenetics: 46XX, der(2)t(1;2),(q21;q37)
Treatment Course:
Phase 1 (Early-Mid 2023):
- Started on CyBorD (Cyclophosphamide, Bortezomib, Dexamethasone)
- Required hemodialysis for AKI
- Improved enough to transition to ambulatory care
Phase 2 (Mid 2023):
- Switched to VTd (Bortezomib, Thalidomide, Dexamethasone)
- Completed 4 cycles
Phase 3 (Late 2023):
- Changed to KPD regimen
- Achieved Complete Response (CR) after 3 cycles
Phase 4 (January 2024):
- Underwent Autologous Bone Marrow Transplant
- Started pomalidomide maintenance in April 2024
Current Status (December 2024-January 2025):
1. Disease Status:
- Biochemical progression on pomalidomide
- M band present
- K/LAMBDA ratio: 6044
- LDH: 885
2. Current Labs (11.1.25):
- Hb: 8 g/dL
- TLC: 4400
- Platelets: 35k
- Creatinine: 5.5
- Urea: 134
3. Current Treatment:
- Started CPd (Cyclophosphamide + Pomalidomide + Dexamethasone) on 31.12.24
- Treatment Protocol:
- Pomalidomide 4mg D1-21
- Cyclophosphamide 500mg D1,D8,D15,D22
- Dexamethasone 40mg D1,D8,D15,D22
4. Supportive Care:
- Ecosprin 75mg (anticoagulation)
- Acyclovir 200mg BD (antiviral prophylaxis)
- Salbutamol nebulization
- Regular nephrology follow-up
This case demonstrates:
1. High-risk myeloma management
2. Complex treatment sequencing
3. Management of renal complications
4. Importance of maintenance therapy
5. Handling of disease progression after transplant
6. Role of supportive care in management
The patient's journey illustrates the typical progression of high-risk multiple myeloma, showing initial response, transplant consolidation, maintenance, and eventual progression requiring subsequent lines of therapy.
At admission : ( 14-1-25 )
Patient is asymptomatic
On routine evaluation - platelets 7000 cells
1 unit SDP transfusion done
In view of severe thrombocytopenia Cyclophosphamide and pomalidomide was withholded as per medical oncologist advice.
Admission 2 :
Patient presented with chief complaints of dark coloured patches on forearm since -4 days and worsening cough and SOB since a week.
soap update date 2 :

 Well your patient is currently on cyclophosphamide and pomalidomide, and a significant side effect that can occur with this combination is thrombocytopenia, this is considered one of the most common grade 3 or 4 hematological adverse events associated with this combination therapy, often requiring close monitoring during treatment. https://pmc.ncbi.nlm.nih.gov/
Well your patient is currently on cyclophosphamide and pomalidomide, and a significant side effect that can occur with this combination is thrombocytopenia, this is considered one of the most common grade 3 or 4 hematological adverse events associated with this combination therapy, often requiring close monitoring during treatment. https://pmc.ncbi.nlm.nih.gov/ https://www.sciencedirect.com/
https://www.sciencedirect.com/











Comments
Post a Comment